Chelation Therapy NYC - Dr. John Salerno
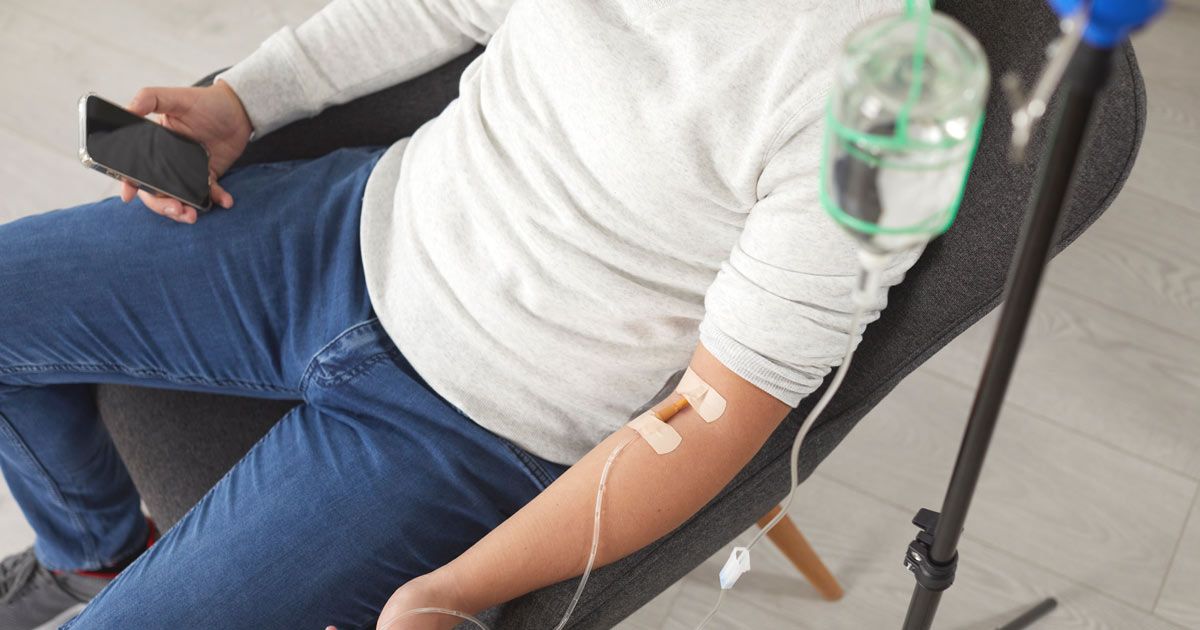
Contact Dr. John Salerno of Salerno Wellness for a Chelation Therapy Consultation in Manhattan, NY, and Connecticut
What is Chelation Therapy?
Chelation therapy is a medical treatment originally developed to remove heavy metals from the body. It involves the administration of chelating agents, chemical substances that have the ability to bind to metals like lead, mercury, and arsenic, facilitating their excretion from the body. While traditionally used for treating heavy metal poisoning, chelation therapy has been effective as a treatment for other conditions. Sessions of chelation therapy typically involve intravenous infusions of the chelating agent over a period of time, with the specific regimen depending on the individual's health needs and the levels of metals present in their body.
Watch Dr. John Salerno of Salerno Wellness talk about treating a patient with heavy metal poisoning from her makeup on Fox 5 News.
Types of Heavy Metal Poisoning
Heavy metal poisoning can result from exposure to a variety of toxic metals. While the list below focuses on the primary metals known for their toxicity and prevalence in causing health issues, it is not exhaustive, as different metals may pose risks depending on exposure levels and individual susceptibility. Below are the most common metals associated with heavy metal poisoning:
- Lead: Used in batteries, old pipes, paints, and various industrial products.
- Mercury: Found in certain fish, dental fillings, and industrial emissions.
- Cadmium: Present in rechargeable batteries, electroplating, and cigarette smoke.
- Arsenic: Occurs naturally in some water supplies and is used in pesticides and preservatives.
- Chromium: Exists in different forms, some of which are used in industrial processes like chrome plating.
- Nickel: Found in stainless steel, coins, and industrial applications.
- Thallium: Previously used in rodenticides and found as a byproduct in various industries.
- Aluminum: Although not traditionally categorized as a heavy metal, it can be toxic in certain circumstances and is present in some food additives and antiperspirants.
- Cobalt: Used in alloys and certain types of industrial processes.
- Manganese: Necessary in trace amounts but toxic at higher exposures, particularly in industrial settings.
- Beryllium: Used in aerospace and manufacturing industries, can be hazardous through inhalation.
- Uranium: Radioactive and chemically toxic, found in nuclear materials and certain rocks and soils.
These metals can accumulate in human tissues and organs over time, leading to various health issues such as neurological damage, kidney dysfunction, and other systemic effects, depending on the type and duration of exposure.
Dr. John Salerno's Advanced Heavy Metal Medical Testing
The Heavy Metal Challenge Test is a method to assess whether chelation therapy is necessary by evaluating the levels of heavy metals present in your body. This thorough test measures over 15 toxic metals stored in tissues and organs over a lifetime, providing a detailed insight into your heavy metal exposure. Initially, a chelating agent is administered; this compound binds to metals such as lead, mercury, cadmium, and arsenic, facilitating their removal through natural detoxification pathways.
Following the administration of the chelating agent, urine samples are collected over a six-hour period, as heavy metals are excreted via urine. These samples are sent for detailed lab analysis to measure the levels of different heavy metals present in your system. Once the lab provides its results, a doctor evaluates them to determine if there are elevated levels indicating heavy metal toxicity and if further intervention is needed.
Based on this analysis, your doctor may decide on specific treatments, which could include additional chelation therapy sessions or alternative strategies to decrease heavy metal levels, thereby enhancing your health. Regular follow-ups are an essential part of the process, ensuring that metal levels are effectively reduced and necessary adjustments to your treatment can be made for continued improvements in your overall well-being.
Medical Conditions Our Chelation Therapy NYC and CT Treat
- Heavy Metal Toxicity - Chelation Therapy is a proven method for removing heavy metals such as mercury, lead, and arsenic from the body. These toxic metals can contribute to various health concerns, including fatigue, cognitive issues, and immune dysfunction.
- Cardiovascular Disease - Chelation Therapy can help reduce arterial plaque, improve blood flow, and alleviate symptoms of conditions such as atherosclerosis and coronary artery disease. It’s a valuable complementary approach for improving heart health.
- Diabetes Complications - Chelation Treatments improve circulation and reduce inflammation, offering potential relief for diabetes-related complications like neuropathy and vascular issues.
- Autism Spectrum Disorder (ASD) - Patients with Autism Spectrum Disorder may experience heavy metal toxicity, which could exacerbate certain symptoms. Medical Chelation Treatments are used as an adjunctive approach to help detoxify the body and reduce metal burden, supporting overall neurological health and function.
- Chronic Fatigue Syndrome (CFS) - Chelation Therapy eliminates toxins that may contribute to fatigue, helping individuals with CFS regain energy and vitality.
- Neurodegenerative Conditions - Chelation Therapy supports brain health by addressing oxidative stress and heavy metal buildup associated with conditions like Alzheimer’s disease, Parkinson’s disease, and other neurological disorders.
- Autoimmune Diseases - By lowering the toxic burden on the immune system, Chelation Therapy can provide relief for autoimmune conditions such as lupus and rheumatoid arthritis.
- Peripheral Vascular Disease (PVD) - Chelation Therapy enhances circulation by removing calcifications and heavy metals that impede blood flow, helping individuals with PVD.
- Skin Conditions - Toxins in the body can contribute to chronic skin problems like eczema, psoriasis, and acne. Chelation Therapy aids detoxification, promoting healthier skin.
- Prevention of Premature Aging - Chelation Therapy reduces oxidative stress and improves cellular health, which can help slow the aging process and promote overall vitality.
What Can Happen if Heavy Metals Are Left Untreated?
If a heavy metal detox is not performed when necessary, the body may continue to accumulate harmful metals, leading to a variety of serious health problems. Over time, this accumulation can affect vital organs, including the liver and kidneys, impairing their ability to filter and eliminate toxins. Neurological issues can arise, as metals like lead and mercury are known to disrupt cognitive functions and may contribute to memory loss, headaches, and mood disorders. The immune system can also become compromised, making individuals more susceptible to infections and illnesses.
People may experience chronic fatigue, unexplained pain, and digestive issues, all of which can severely impact daily life. There is also a risk of developing autoimmune diseases, as heavy metals can trigger inflammatory responses in the body. Hormonal imbalances may occur, leading to reproductive issues and other endocrine-related problems. Additionally, exposure to heavy metals has been linked to an increased risk of certain cancers, further underscoring the danger of neglecting detoxification.
Long-term exposure can also interfere with metabolic processes, affecting nutrient absorption and leading to deficiencies that complicate overall health. Mental health can deteriorate as well, with increased anxiety, depression, and behavioral changes potentially linked to heavy metal toxicity. Ultimately, the failure to detoxify can create a toxic burden that diminishes quality of life, making it crucial to address heavy metal exposure proactively. The longer one waits to cleanse the body of these toxic substances, the more challenging recovery may become. Ignoring the need for detoxification can thus lead to a cascade of health issues that could potentially be avoided with timely intervention.
Case Study: Chelation Therapy for Heavy Metal Detoxification
Patient J.L., a 52-year-old software engineer, had been experiencing a range of inexplicable symptoms over several months, including persistent fatigue, joint pain, and cognitive difficulties. Despite consultations with various specialists, J.L. found no relief, and his symptoms continued to impact his daily life and work performance. Eventually, J.L. was referred to Dr. John Salerno, a physician renowned for his expertise in integrative medicine and chelation therapy.
Upon initial evaluation, Dr. Salerno conducted a comprehensive battery of tests, including a heavy metal challenge test. The results indicated significantly elevated levels of mercury and lead, suggesting that heavy metal toxicity could be contributing to J.L.’s health issues. Dr. Salerno recommended a tailored chelation therapy regimen to address these imbalances and help detoxify J.L.’s system.
The treatment plan involved regular sessions over the course of several months, during which J.L. received intravenous infusions of a chelating agent known as EDTA. These sessions were carefully monitored to manage any potential side effects, including dehydration and nutrient depletion, which were mitigated through supplemental vitamins and minerals. Dr. Salerno encouraged J.L. to adopt a diet rich in organic foods, antioxidants, and fiber to support the body’s natural detoxification processes and enhance the effectiveness of the therapy.
Remarkably, within the first weeks of treatment, J.L. reported notable improvements in his energy levels and cognitive clarity. Over the subsequent months, his joint pain subsided significantly, enabling him to resume his physical activities and engage more actively at work. Regular follow-up appointments ensured that J.L.’s progress was closely tracked and any necessary adjustments to the therapy were made.
By the end of the chelation therapy program, J.L.’s heavy metal levels had decreased substantially, and his symptoms had dramatically diminished. He expressed profound gratitude to Dr. Salerno, crediting the treatment with restoring his quality of life and enabling him to focus on his career and personal pursuits. This case highlights the potential for chelation therapy, under expert guidance, to alleviate symptoms associated with heavy metal toxicity and enhance overall well-being.
Contact Dr. John Salerno of Salerno Wellness for a Chelation Therapy Consultation
Are you ready to take charge of your health? Discover the transformative benefits of Chelation Therapy with Dr. John Salerno at Salerno Wellness. Our tailored approach can help remove harmful toxins from your body and improve your overall well-being. Don’t wait any longer—schedule your personalized consultation today and start your journey towards optimal health! Contact us now to take the first step—your wellness deserves it!
Please contact us today.
Additional References
- Chelation for Coronary Heart Disease: What You Need To Know - National Center for Complementary and Integrative Health
- Chelation Therapy - Cleveland Clinic



